Discover how to develop your own hyaluronic acid-based medical device line: production processes and standards for safe, effective, certified products.
The production of hyaluronic acid-based medical devices requires high standards of quality, safety, and innovation. Every stage must be carefully monitored, from the fermentation of raw materials to final packaging.
In this article, we explore every step of the production process, the key criteria for selecting a contract manufacturing partner, and what sets the best products on the market apart.
Whether you’re planning to launch a custom product line or simply want to understand how these innovative devices are made, keep reading to discover everything about hyaluronic acid medical device production.
The production of hyaluronic acid injectables involves advanced technologies to ensure safety and efficacy.
The process begins with bacterial fermentation, which generates a highly pure and biocompatible substance. This initial stage is essential to guarantee the quality of the final product.
After fermentation, the hyaluronic acid is purified to remove any residues. This is followed by crosslinking, a process that enhances its stability and longevity. These steps take place in controlled environments to maintain high standards.
Filling is carried out in ISO-certified clean rooms using automated machinery that minimizes contamination risks.
As all stages comply with medical device manufacturing standards, each batch is tested for sterility, viscosity, and pH—ensuring it meets international quality requirements. Complete traceability and rigorous quality control make hyaluronic acid injectables safe for professional use in aesthetic medicine.
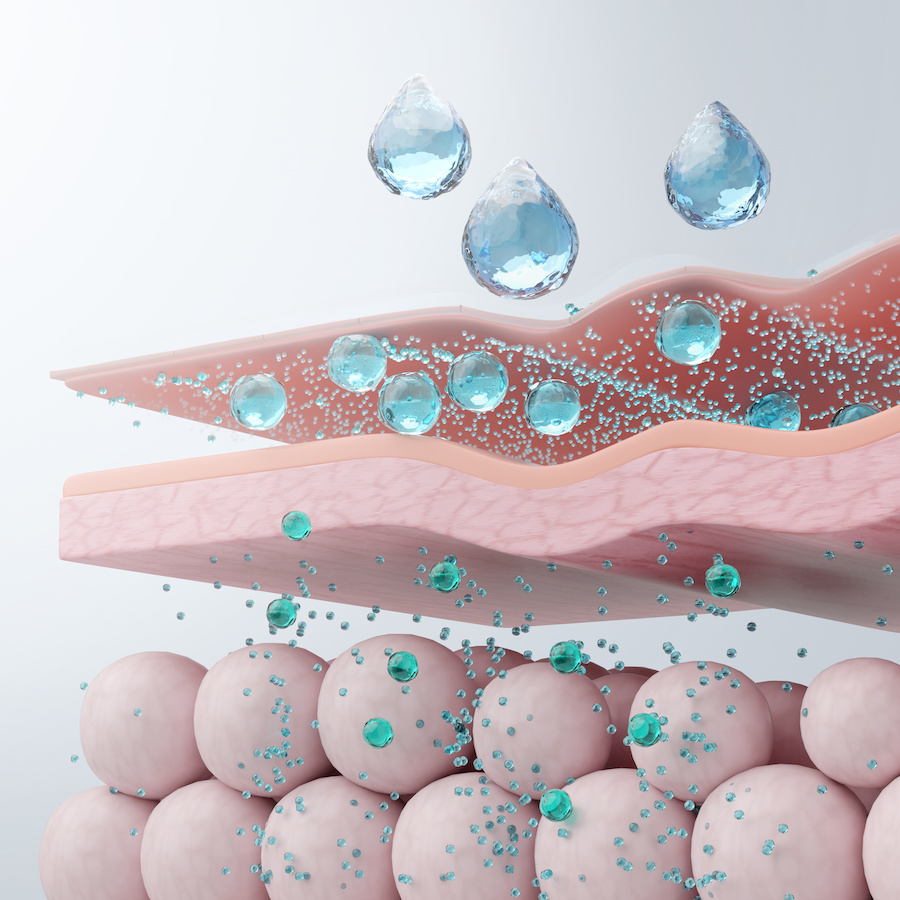

Outsourcing the production of hyaluronic acid injectables requires collaboration with specialized partners. These providers must be experts in medical device manufacturing and operate certified laboratories. Choosing the right supplier is crucial for the quality and safety of the final product.
The process includes formula development, material selection, filling, and packaging. Every step must comply with ISO and GMP standards to ensure traceability and regulatory compliance. The resulting medical device must obtain CE certification before entering the market.
Phitogen offers a full-service solution for the production of Class III medical devices, overseeing every phase in high-tech environments that use eco-sustainable processes. The company ensures quality, innovation, and adherence to European regulations, delivering tailor-made solutions based on fermented and purified hyaluronic acid.
This integrated approach guarantees compliant, safe, and effective hyaluronic acid products ready for medical and aesthetic use. Partnering with an experienced manufacturer reduces development time and optimizes costs, streamlining market entry with high-quality products.
Phitogen’s hyaluronic acid stands out thanks to its Conservative Cross-Linking technology—an innovative process that ensures precise control of crosslinking, prevents depolymerization, and uses less BDDE.
The result is a smooth, easy-to-handle, and safe product, designed to deliver a natural and long-lasting effect.
The Research & Development division collaborates with international experts in medical device production. The team continuously refines techniques and formulas to ensure high performance, certified quality, and excellent tissue compatibility. Every stage is designed to create effective, well-tolerated devices.
Phitogen’s hyaluronic acid penetrates deeply and integrates seamlessly with the skin.
This ensures a gradual rejuvenation effect without altering natural facial features. The product enhances skin elasticity and activates visible repair processes over time.
Phitogen develops cutting-edge medical devices that combine scientific research with sustainability. The final result is authentic, enhancing each person’s genetic uniqueness while promoting skin health.
Made in Italy production
Highest quality raw materials
Technological innovation, research and development
Innovative formulas certified
Phitogen Beauty Labs
La principale di un gruppo di imprese focalizzate, da oltre vent’anni, nella produzione conto terzi e distribuzione di medical device a base di Acido Ialuronico, impiegati in medicina estetica, ortopedia e dermocosmetica.
Contatti
Via Valtellina, 21
San Benedetto del Tronto (AP)
63074 - Italia
Tel: +39 0735 762020
Email: export@phitogen.it


